Surfactants (short for surface active agent) are molecules used to alter interfacial properties by adsorbing at the boundary between two immiscible phases, and play an important role in many industrial applications. A particular situation where surfactants are vital to effective operation is flow assurance in oil and gas extraction, where gas hydrates (inclusion compounds that consist of water and small hydrocarbon molecules) can form spontaneously under common operation conditions, causing pipeline and equipment blockages that can yield catastrophic effects. To prevent the formation of hydrate plugs, a specific class of surfactants (so-called anti-agglomerants) is added to the oil-water-gas mixture flowing through the extraction pipelines. The anti-agglomerants adsorb on the initial microscopically small hydrate crystals and inhibit their agglomeration into larger compounds. It is crucial to improve the effectiveness of the currently used anti-agglomerants to lower the needed dosage and to better fulfill environmental regulations.
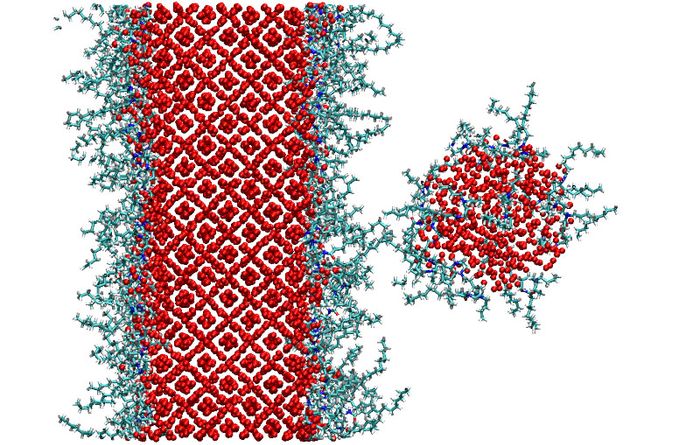
In collaboration with Clariant Oil Services and Clariant Produkte (Deutschland) GmbH, one of our industrial partners, researchers from Nextmol have used molecular modeling techniques to investigate four anti-agglomerant molecules with respect to their capability to prevent the formation of hydrate plugs. The computational simulations have been carried out using both steered and non-steered molecular dynamics (MD), simulating the coalescence process of a hydrate slab and a water droplet, both covered with anti-agglomerants. Based on these calculations we have established a ranking of the molecules according to free energy calculations (steered MD) and the number of agglomeration events (non-steered MD). In other words, we have predicted which surfactants are expected to perform well and badly as anti-agglomerants, respectively.
Subsequently, the same molecules have been tested experimentally by our partner. Based on rocking cell measurements, they have determined the minimum effective dose necessary to inhibit agglomeration. This again has allowed to rank the surfactants according to their anti-agglomeration power. We have observed an excellent agreement between the obtained experimental ranking and the ranking predicted by our simulations.
These results indicate that our simulations are capable to accurately predict the performance of such surfactants to inhibit the agglomeration of gas hydrate particles. Moreover, the molecular simulations provide additional insights into the agglomeration process and the way in which the anti-agglomerants prevent it that would be not accessible with purely experimental methods. For instance, we have analyzed in this study the density profiles at the interface, the diffusion of the anti-agglomerants, and the orientation of their tails.
The possibility to perform systematic computational high-throughput screenings of many molecules, exploiting scalable computational resources, enables to set up an efficient funnel approach where only the most promising candidates will eventually be synthesized and tested in the lab. This allows to go beyond a purely experimental approach where one has to synthesize and test every molecule in the laboratory, making thus research more efficient and scalable.
This work has been published in The Journal of Physical Chemistry B. Contact us for more information.